Preparation & administration
Choosing the appropriate supplies
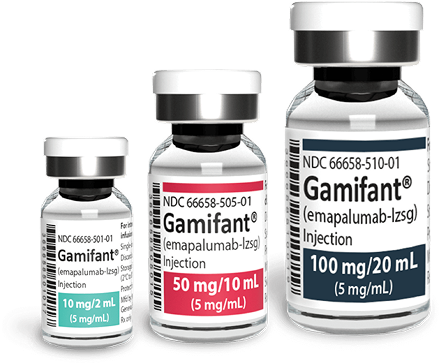
Selecting your vials1
Gamifant is available in 10 mg/2 mL, 50 mg/10 mL, and 100 mg/20 mL vials. Please note that vials are single dose only. Any remaining drug must be discarded.
Select the appropriate bags or syringes1
Depending on the patient’s weight and dose, the diluted sterile concentrate can be administered in:
- 20 mL or larger syringes
- 0.9% sodium chloride for injection, USP infusion bag of the appropriate size, depending on the volume to be infused.
Note: Gamifant can be administered using a gamma-irradiated, or ethylene oxide–sterilized, latex-free, polyvinyl chloride (PVC)-free syringe, or a non-PVC polyolefin infusion bag (dependent on volume needed).
Prepare the Gamifant dilution1
1
Remove Gamifant from the refrigerator and inspect vials for particulate matter and discoloration. Gamifant should be clear to slightly opalescent, and colorless to slightly yellow. Do not use if it is discolored or if foreign particulate matter is present.
2
Withdraw the necessary amount of Gamifant solution and dilute with 0.9% sodium chloride injection, USP, to a maximum concentration of 2.5 mg/mL. Do not dilute product to less than 0.25 mg/mL.
3
Discard any unused portion left in the vial(s). Gamifant vials are for single use only.
4
Place the diluted solution in a syringe or infusion bag.
Gently invert the infusion bag or syringe several times to ensure complete and homogeneous distribution of Gamifant.
DO NOT SHAKE
5
Once the infusion solution is prepared, it should clearly be labeled for administration to the patient.
Administration of Gamifant1

- Administer Gamifant diluted solution over 1 hour through a central or peripheral intravenous line containing a sterile, non-pyrogenic, low–protein binding, 0.2-μm in-line filter
- Do not store any unused portion of the infusion solution for reuse. Any unused product or waste material should be disposed of in accordance with local requirements
- If you do not administer Gamifant immediately, download the Dosing and Administration Guide for storage information