Real-World Evidence
Real-world evidence in primary HLH: the REAL-HLH study1
A retrospective review of patients with primary HLH treated with Gamifant
REAL-HLH is the first real-world study to describe the clinical characteristics, treatment patterns, and outcomes of Gamifant treatment across a large and diverse population of patients who have primary hemophagocytic lymphohistiocytosis (HLH).
Study design1
A retrospective medical chart review was conducted across 33 US hospitals to identify patients treated with ≥1 dose of Gamifant between Nov 20, 2018, and Oct 31, 2021.
Given the complexity of the disease and risk for misclassification, a consistent rule was applied across all enrolled HLH patients and reviewed by a multispecialty panel of experts. Patients were classified as having primary HLH if they met at least 1 of 3 of the following criteria without evidence of underlying malignancy, rheumatologic, or metabolic disease: at least 5 of 8 HLH- 2004 diagnostic criteria OR a family history of HLH OR a known genetic mutation associated with primary HLH.
HSCT=hematopoietic stem cell transplantation.
*The REAL-HLH study was a retrospective, noninterventional medical chart review and was not evaluated by the FDA to grant Gamifant approval. Data were extracted from time of Gamifant initiation to end of data availability, end of study (Dec 31, 2021), or death, whichever occurred first.
Demographics1
46
patients were included in the analysis age range: 0.3-21 years old, median: 1 year
39 children 0.3-10 years old
7 adolescents and adults 12-21 years old
90
(27/30) had ≥5 out of 8 HLH-2004 diagnostic criteria
92% (23/25)
80% (4/5)
54.3
(25/46) had active infections† at diagnosis
51.3% (20/39)
71.4% (5/7)
21.7
(10/46) had CNS involvement at diagnosis
23.1% (9/39)
14.3% (1/7)
†Viral infections were the most common (72%, 18/25).
empty on purpose
Dosing1
‡The recommended starting dose of Gamifant is 1 mg/kg given as an intravenous infusion over 1 hour twice per week.2 Click here for dosing information.
Results
Limitations of analysis:
Due to the study design, information could be missing or incomplete, as data may not be uniformly collected or available across all treatment centers. The constrained availability and inconsistent timing of laboratory assessments further hindered comprehensive evaluation of treatment "response." Additionally, safety data were neither collected nor assessed since the study did not incorporate safety-related endpoints. There may be the possibility of a risk of bias toward patients with poor prognoses, as Gamifant is currently indicated for previously treated patients with primary HLH. As patients were concomitantly administered HLH-related therapies, these findings cannot be solely attributed to Gamifant and may not be generalizable beyond the study cohort. The rarity of primary HLH limited the size of the population in this study and the types of analyses that could be performed, particularly when comparing outcomes between children and adolescents/adults.
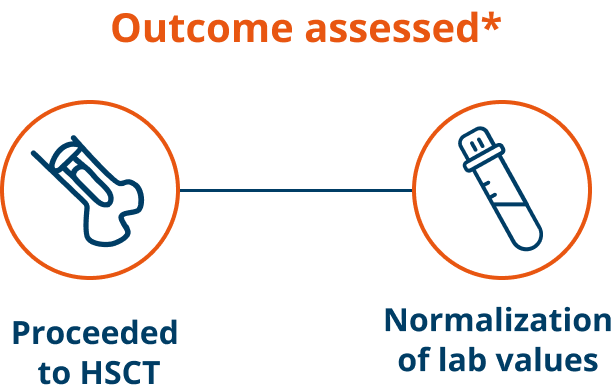
A majority of patients proceeded to HSCT1
(31/42) of patients treated who were eligible for transplant proceeded to HSCT
Time to normalization of laboratory parameters1
The top 5 laboratory parameters for which most of the patients achieved normalization were fibrinogen, absolute neutrophil count, platelets, absolute lymphocyte count, and alanine transaminase.
Select the parameter on the graph below that you would like highlighted.§,‖
CXCL9
72.7% patients (24/33)
28.5 days (range: 4-84 days)3
CXCL9=chemokine (C-X-C motif) ligand 9; sCD25=soluble cluster of differentiation 25.
§Laboratory parameters for which data were available for ≥50% of the study population.
‖Normalization of laboratory parameters and biomarker values were based on physician report.
The generalizability of reported results may not correlate with results seen in other real-world setting patients. Continuous variables were summarized using mean, standard deviation, median, and interquartile range. Categorical variables were described using counts and percentages.
Laboratory parameter normalization breakdown
Fibrinogen
97.4% patients (37/38)
14 days (range: 1-91 days)3
Absolute neutrophil count
88.9% patients (40/45)
7.5 days (range: 3-63 days)3
Platelets
84.8% patients (39/46)
8 days (range: 3-76 days)3
Absolute lymphocyte count
71.4% patients (30/42)
7 days (range: 3-230 days)3
Alanine transaminase
68.9% patients (31/45)
26 days (range: 2-113 days)3
CXCL9
72.7% patients (24/33)
28.5 days (range: 4-84 days)3
sCD25
54.1% patients (20/37)
12.5 days (range: 2-84 days)3
Ferritin
44.4% patients (20/45)
21.5 days (range: 4-112 days)3
The authors concluded that the results from the REAL-HLH study are consistent with the pivotal trial for Gamifant across age groups.