
Now
approved
for Macrophage
Activation
Syndrome
(MAS) in Still's Disease1,2
Gamifant is the first FDA-approved treatment for patients with HLH/MAS in known or suspected Still's disease, including systemic Juvenile Idiopathic Arthritis (sJIA), with an inadequate response or intolerance to glucocorticoids, or with recurrent MAS.
Read the press release forthe FDA approval
of Gamifant
for MAS in Still's Disease
The latest Example of Sobi's
commitment to treating HLH
Gamifant's approval in MAS is the latest advancement in the treatment of HLH. Gamifant received its first FDA approval in 2018 for the treatment of adult and pediatric (newborn and older) patients with primary hemophagocytic lymphohistiocytosis (HLH) with refractory, recurrent, or progressive disease or intolerance with conventional HLH therapy.1,2
Gamifant is an interferon gamma (IFNγ)-blocking antibody that targets a key mediator of hyperinflammation, which drives the life-threatening symptoms of primary HLH and MAS in Still's disease.1-4
As Sobi remains dedicated to providing access to innovative treatments, we are pleased to announce that this new indication for patients with MAS in Still's disease is now the first FDA-approved treatment option for MAS in Still's disease, meeting an unmet need for patients.1,2
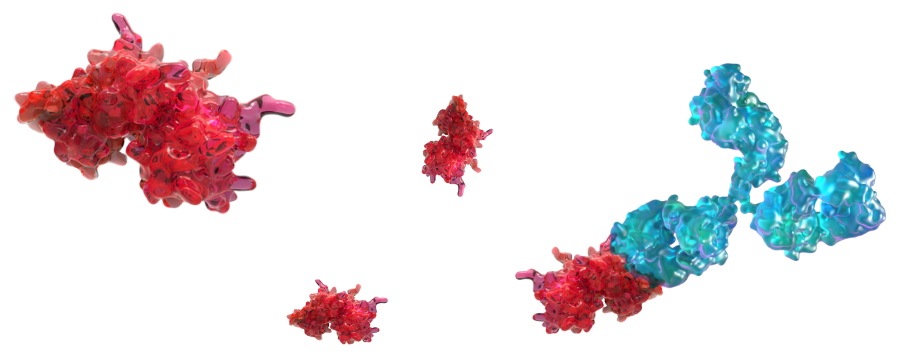

Gamifant targets IFNγ—
a key cytokine In MAS In
Still's Disease
IFNγ is a central and upstream mediator of the hyperinflammatory feedback loop in MAS with Still's disease. IFNγ is a key “macrophage-activating factor,” increasing cytokine and chemokine production and phagocytosis in a continuous feedback loop.3
Gamifant is a monoclonal antibody that binds with affinity to free and receptor-bound IFNγ, neutralizing its activity.1,4

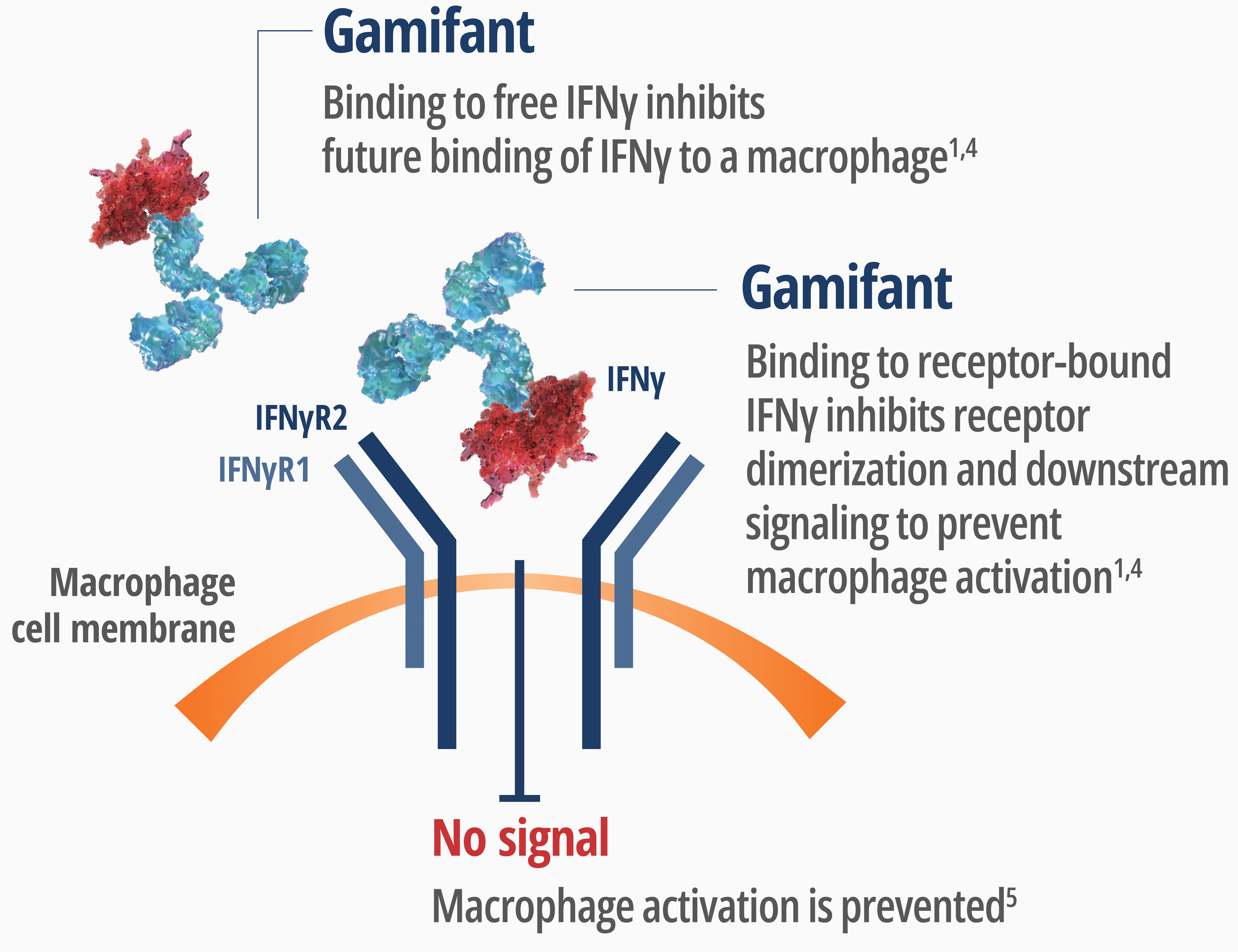
Gamifant binds to free and receptor-bound forms of IFNγ1,4

This neutralizes IFNγ activity and blocks intracellular signaling1,4

Hyperactivation of macrophages by excess IFNγ is prevented1,6

Hyperinflammation is subdued1,4
Contact us
Interested in more information about treatment with Gamifant and MAS in Still's disease? Reach out and get your questions answered by a Gamifant Representative today.
Contact Sobi now
Ordering Gamifant
There are 2 pathways to access Gamifant. Keep in mind that your institution and the patient's insurance will dictate how Gamifant should be ordered.
Download the ordering guide
Specialty Pharmacy (SP)
Phone: 800-850-4306, option 2
Fax: 800-823-4506
INPATIENT OR OUTPATIENT
Patient pays out of
pocket to SP

Plasma and Biologics
Specialty Distributor
Phone: 877-625-2566
Fax: 888-752-7626
connect.mckesson.com
INPATIENT
Patient pays out
of pocket to hospital
OUTPATIENT
Patient pays out of pocket to infusion center/physician practice

Personalized support
for patients
Gamifant Cares offers access and reimbursement support to help patients access Gamifant. Gamifant Cares provides information regarding patient healthcare coverage options and financial assistance information that may be available to help patients with financial needs.

